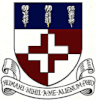As anaesthetists, we are dependent upon information. Information about physical properties (temperatures, pressures), chemical properties (concentrations), rates of change (frequencies) and many others. Whilst it is easy to say that a surface is warm or cold, that is rarely of much use ! We want to be able to associate the information with some form of scale and then to display that information in a form which we can use.
That simple specification, involves some quite complicated ideas.
Firstly, we require a device whose properties change in a predictable and measurable way in response to what we want to measure. Since we have become quite good at manipulating electrical signals, this will usually mean that we want some sort of transducer which produces an electrical output. By way of example, consider the oxygen fuel cell (full details below, but for the time being, consider it as a battery whose output voltage is dependent upon oxygen concentration). Having built ourselves a fuel cell, we have a device which produces a changing electrical output in response to a changing oxygen concentration. But what does any specific voltage signify ?
For a tranducer with a linear response (i.e. which fits with the 'general equation to a straight line' y=mx+c):
- Zeroing
- Linerity
- Drift
Zeroing sets the intercept with the y-axis. In reality, 'zero' may not represent a realistic measurement option (could we zero our thermometers at 0°K) ? Instead another, fixed point is used, sucha as 0°C (273°K). This is a one-point calibration.
Performing a two-point calibration allows the gradient 'm' to be calculated. This requires a second calibration point - we can calibrate our fuel-cell in 100% oxygen.
Correction for drift comes by repeating the above calibrations after a set interval. How long this will be will depend on the tranducer (ABG machine will perform very regular one and two-point calibrations, whereas an oxygen fuel cell will only be recalibrated every 24hrs. For a more detailed review, see the monitoring seciont in the physics section.
ECG
The ECG has a signal amplitude of around 1mv (typical range 0.01-5mV). Such a small signal must be amplified, but this creates two problems:
- DC offset - when two substances with different electrochemical properties are in contact, a potential difference exists at the juntion. Simply connecting an ECG electrode to the skin (metal contact with an electrolyte gel) creates a 'battery'. Careful design allows this to be minimised, but as the ECG signal is amplified, so is this DC offset. As a result, the ECG signal soon vanishes off the Y-axis of the monitor screen ! If only we had a component which could block DC, but pass an AC signal...
- Interference - the ECG consists of a range of frequncies in the range 0.05-100Hz, but the bulk of this is in the range 0.05-35Hz. The theatre environment contains a great deal of electical 'noise'. We are surrounded by mains wiring (50Hz current) and the surgeons often use diathermy (10-100Mhz). This noise is amplified along with the tiny ECG signal and may well swamp it. Since the bulk of the electrical noise occurs at frequencies higher than those of the ECG, it would be useful to have a component which tended to block the passage of AC currents.
The combination of inductors and capacitors allows the creation of a filter which will tend to pass a narrow range of frequencies. However, there is no perfect configuration ! During surgery we may choose to have very heavy filtration of the high, diathermy frequencies BUT this caomes at a cost. Pacing spikes are also high-frequency signals which are quiclky filtered out. As a result, you will often find that your ECG config offer options such as 'diagnostic', 'monitoring' and 'surgery'. These correspond with low, medium and high levels of filtration. The low-level shows the signal in its entirity, but may include so much noise as to be useless. Alterntaively, the highly filtered signal may be useful in allowing an ECG signal to be seen during surgery, but some of the detail may be missing.
To explore these ideas, play with the 'components board'.
ECG electrodes consist of a silver contact with a silver-chloride impregnated gel layer.
Gases
Gas concentrations can be determined by exploiting their chemical or physical properties. Since an anaethetic circuit contains a mixture of compounds there is the possibility of using one device to measure several components OR for the accuracy of a measurement to be disturbed by the presence of another agent.
Amongst the physical properties used are:
Beer-Lambert law
Beer's law states that the intensity of transmitted light decreases exponentially as the concentration of a substance increases.
Lambert's law states that the intensity of transmitted light decrease exponentially as the distance travelled through the substance increase
Combining these two gives:
A = log10 Ii/It = εlC
The beer-Lambert law.
A-absorbance, Ii-incident light intensity, It-transmitted light intensity, ε-the extinction coefficient, l-path length and C-concentration. YouTube explanation
- Absorption of light at specific wavelengths. The inter-atomic bonds between different atoms can absorb energy. The energies involved correspond with photons in the infra-red (IR) part of the spectrum. Since this applies to gases with different atoms, it does not apply to oxygen or nitrogen, but can be used with CO2 or N2O. Water vapour will also absorb IR light and this may interfere with the measurement. Poly-atomic compounds such as the volatile agents, can absorb at several wavelengths which makes it possible to determine which agent it present and in what concentration. This approach depends on the Beer-Lambert law (equation 1).
- Scattering of light - a tiny proportion of light hitting or passing through a substance can interact in such a way as to change its frequency. This shift is characteristic of the substance and its concentration as is know as Raman scattering.
- Magnetic attraction - most gases are repelled by a magnetic field (these are termed diamagnetic, but some are attracted (paramagnetic).
- Refractive index - not used clinically but important because it is highly accurate and applicable to all gases. Used by manufacturers as one option for calibrating equipment (e.g. vaporizers).
- Molecular weight and charge when ionised - this can be applied to any compound. By bombarding molecules with electrons, some become ionised and can be accelerated in an electric field. Their velocity will depend on the (constant) electric field strength and mass (the smaller the mass the greater the acceleration). On its own, this just gives a stream of particles, which is not much use ! However, applying a magnetic field will tend to curve the paths of the ioins and the degree of curvature will depend on ratio of charge to mass (highly charged with a small mass gives significant deviation). This flow of charged particles is a current and can be detected, the magnitude of the current at different positions in the detector creating a fingerprint of the parent substance (useful explanation). Mass spectrometers are large and complex and do not have a clinical role.
In order to perform an analysis, we need a sample of gas. This might come from gas in the circuit (main-stream sampling) but this is is often impractical to have the analyser connected to part of the breathing system. Instead, it becomes necessary to remove sample gas from the circuit and transport it to the analyser. This is side-stream analysis. Some of the analytical processes are destructive (e.g. mass spectrometry), but others are not (e.g. absorption spectroscopy), In the latter case, it is possible to return the sample gas to the breathing system.
You may enounter main-stream analysers in resuscitation, where they are oftern used for CO2 measurment.
As the analysers become more sophisticated (larger and heavier), it becomes impractical to have them connected directly to the breathing system and almost all of our anaesthetic agent anaysers use the side-stream approach. This has the disadvantage that it introduces a time delay into the measurements as it takes time for gas samples to be drawn along the sample tubing and reach the machine. When you have a circuit disconnect, it will not be shown immediately !
Oxygen

A chemical fuel cell
There are two types of oxygen analysers found in anaesthetic machines. Fuel cells and para-magnetic analysers.

Galvanic fuel cell
Conceptually, fuel cells are just a form of battery. They require oxygen for an electro-chemical reacation and the more oxygen is available, the higher the voltage they produce. Just with this information, we can predict a lot of their behaviour !
O2 + 2H2O + 4e- → 4OH- (Cathode reaction)
2Pb + 4OH- → 2PbO + 2 H2O + 4e- (Anode reaction)
Galvanic fuel-cell equations
Changes in temperature, minor manufacturing variations and age will mean that the exact voltage produced for any given FiO2 will vary between cells and over the course of a cell's lifetime. We cannot assume that the initial output voltage will represent a specific FiO2 (see 'drift'), nor that a specific change in FiO2 will produce a predicatble change in voltage (see 'linearity'). As a result, a calibration must be undertaken with two different oxygen concentrations. The most easily available are 21% (air) and 100%.
Like any battery, eventually they will run 'flat' and so fail to produce a voltage representative of the oxygen concentration. When this happens, you will find that the indicated oxygen concentration is lower than you expect (e.g. the machine reads 40% when 100% is flowing). A 100% calibration will fail as the machine determines that the voltage from the fuel cell is below the minimum specification. All that remains is to replace the cell. Do not be confused by the fact that a 21% calibration may succeed - this is expected as the exhausted cell can still generate some voltage and if this is within range for 21%, then the machine will accept it.
Paramagnetic
Oxygen is magnetic ! This property is used in the para-magnetic analyser. If sample gas is fend into the chamber, then any oxygen it contains is attracted to the magnetic field. If the 'dumbbell' (fig. 3) contains a non-para-magnetic reference gas, then this tends to be pushed out of the way, creating a turning force which can be measured.
A para-magnetic oxygen analyzer
CO2, N2O and volatiles
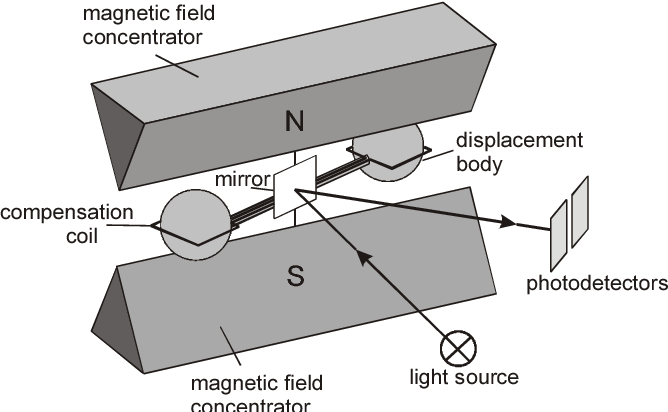
All of these compounds absorb infra-red light and so absorption spectroscopy can be used to measure their concentrations. Using a range of frequencies allows the analyser to determine absorption due to each component in a gas mixture (fig. 4). An example of a real gas-analyser is shown in fig. 5.
A multi-wavelength IR gas analyser
A real gas analyzer
More info